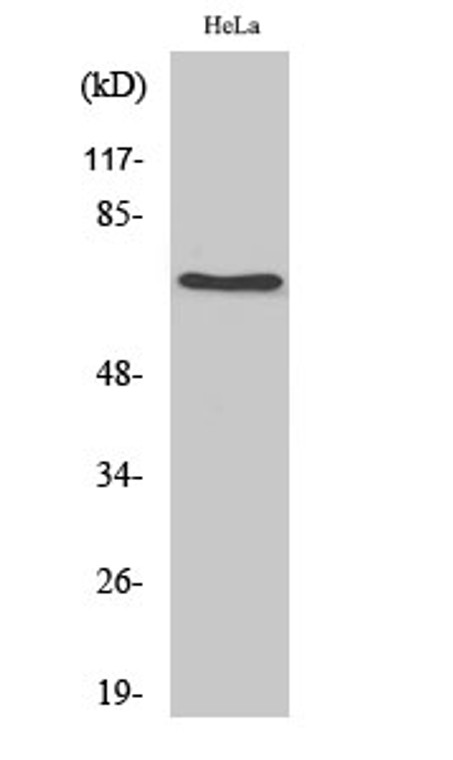
| Host: |
Rabbit |
| Applications: |
WB/ELISA |
| Reactivity: |
Human/Mouse/Rat |
| Note: |
STRICTLY FOR FURTHER SCIENTIFIC RESEARCH USE ONLY (RUO). MUST NOT TO BE USED IN DIAGNOSTIC OR THERAPEUTIC APPLICATIONS. |
| Short Description: |
Rabbit polyclonal antibody anti-Heat shock cognate 71 kDa protein (202-251 aa) is suitable for use in Western Blot and ELISA research applications. |
| Clonality: |
Polyclonal |
| Conjugation: |
Unconjugated |
| Isotype: |
IgG |
| Formulation: |
Liquid in PBS containing 50% Glycerol, 0.5% BSA and 0.02% Sodium Azide. |
| Purification: |
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen. |
| Concentration: |
1 mg/mL |
| Dilution Range: |
WB 1:500-1:2000ELISA 1:10000 |
| Storage Instruction: |
Store at-20°C for up to 1 year from the date of receipt, and avoid repeat freeze-thaw cycles. |
| Gene Symbol: |
HSPA8 |
| Gene ID: |
3312 |
| Uniprot ID: |
HSP7C_HUMAN |
| Immunogen Region: |
202-251 aa |
| Specificity: |
HSC 70 Polyclonal Antibody detects endogenous levels of HSC 70 protein. |
| Immunogen: |
The antiserum was produced against synthesized peptide derived from the human HSC 70 at the amino acid range 202-251 |
| Post Translational Modifications | Acetylated. ISGylated. Trimethylation at Lys-561 reduces fibrillar SNCA binding. |
| Function | Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, chaperone-mediated autophagy, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle of HSP70, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity of HSP70 for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. HSP70 goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The HSP70-associated co-chaperones are of three types: J-domain co-chaperones HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70. Acts as a repressor of transcriptional activation. Inhibits the transcriptional coactivator activity of CITED1 on Smad-mediated transcription. Component of the PRP19-CDC5L complex that forms an integral part of the spliceosome and is required for activating pre-mRNA splicing. May have a scaffolding role in the spliceosome assembly as it contacts all other components of the core complex. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes. Substrate recognition component in chaperone-mediated autophagy (CMA), a selective protein degradation process that mediates degradation of proteins with a -KFERQ motif: HSPA8/HSC70 specifically recognizes and binds cytosolic proteins bearing a -KFERQ motif and promotes their recruitment to the surface of the lysosome where they bind to lysosomal protein LAMP2. KFERQ motif-containing proteins are eventually transported into the lysosomal lumen where they are degraded. Participates in the ER-associated degradation (ERAD) quality control pathway in conjunction with J domain-containing co-chaperones and the E3 ligase STUB1. Interacts with VGF-derived peptide TLQP-21. |
| Protein Name | Heat Shock Cognate 71 Kda ProteinHeat Shock 70 Kda Protein 8Lipopolysaccharide-Associated Protein 1Lap-1Lps-Associated Protein 1 |
| Database Links | Reactome: R-HSA-3371453Reactome: R-HSA-3371497Reactome: R-HSA-3371568Reactome: R-HSA-3371571Reactome: R-HSA-432720Reactome: R-HSA-432722Reactome: R-HSA-447041Reactome: R-HSA-450408Reactome: R-HSA-6785807Reactome: R-HSA-6798695Reactome: R-HSA-72163Reactome: R-HSA-8856828Reactome: R-HSA-8876725Reactome: R-HSA-888590Reactome: R-HSA-9613354Reactome: R-HSA-9613829Reactome: R-HSA-9615710 |
| Cellular Localisation | CytoplasmMelanosomeNucleusNucleolusCell MembraneLysosome MembranePeripheral Membrane ProteinCytoplasmic SideLocalized In Cytoplasmic Mrnp Granules Containing Untranslated MrnasTranslocates Rapidly From The Cytoplasm To The NucleiAnd Especially To The NucleoliUpon Heat Shock |
| Alternative Antibody Names | Anti-Heat Shock Cognate 71 Kda Protein antibodyAnti-Heat Shock 70 Kda Protein 8 antibodyAnti-Lipopolysaccharide-Associated Protein 1 antibodyAnti-Lap-1 antibodyAnti-Lps-Associated Protein 1 antibodyAnti-HSPA8 antibodyAnti-HSC70 antibodyAnti-HSP73 antibodyAnti-HSPA10 antibody |
Information sourced from Uniprot.org
12 months for antibodies. 6 months for ELISA Kits. Please see website T&Cs for further guidance