The History and Future Prospects of Antibodies
Posted by S.Davis on 12th Nov 2020
The History and Future Prospects of Antibodies
It's fair to say that over the past two centuries research and treatment regarding the knowledge and applications of antibodies has come a long way from its early discoveries and basic understandings.
Who first recognised the potential use of antibodies?
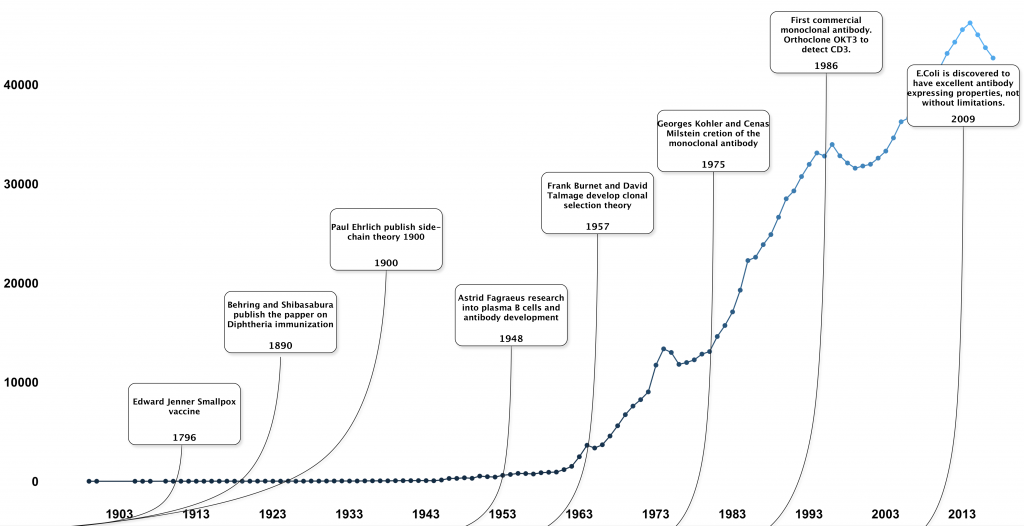
The existence of a component responsible for recognising infections was first brought to light in 1890 by Dr Emil von Behring and his partner Shibasabura Kitasato when the pair produced their landmark publication which demonstrated that the transfer of serum from animals immunised against Diphtheria into animals which were suffering from it could help cure them. So, it's thanks to this publication that specialised research began in order to have an understanding of the components within the immune system which could be responsible for identifying infectious pathogens.
Vaccines have been around longer than you might expect
However, just because the depths of the biochemistry were yet to be understood, vaccinations had been in practice for centuries prior to this in the far east. Scientists and medical practitioners were unaware of the existence of antibodies at this time but the first immunisation process is recorded to have taken place in China around 900AD.
Early vaccination development
The principle of vaccination and immunisation developed by the Chinese is similar to how modern vaccines work, but early day vaccinations posed more risks that what we have today. The method was to deliberately cause a mild infection with unmodified pathogens. This was known as variolation, named after the Variola virus which was used as the infective agent and was practiced through the 14th to 17th century.
Smallpox, the great breakthrough
The most well-known breakthrough in immunization records and vaccinology would be Edward Jenner’s smallpox vaccine in 1796. His vaccine against smallpox was developed by inoculating a boy with the fluid from a cowpox pustule, which gave the boy immunity to the similar but much more aggressive smallpox.
Behring and Kitasato’s publication of serum transfer was the cascade of the development of many antibody theories, but in 1900 it was Paul Ehrlich who became the man responsible for the proposition of the side-chain theory in which he hypothesised that there are side chain receptors on cells that bind to a given pathogen. It is for this reason that the immunology community often refer to Ehrlich as the father of modern immunology despite Behring and Kitasato pioneering the beginning of antibody discovery. But before these two the community only knew how but not the why.
Antibodies in modern times
Fast forward in time to 1948, Dr Astrid Fagraeus researched into B cells and described how plasma B cells played a specific involvement with antibody development. This was further expanded on by Frank Burnet and David Talmage in 1957 when the two developed the clonal selection theory.
The theory
The clonal selection theory of antibodies states that a B lymphocyte makes single specific antibody molecules that are determined before it encounters the antigen.
The leap forward
The 1970s included some of the biggest leaps in modern history with the progression of antibody knowledge and research prospects. With the first atomic structure of an antibody fragment published in 1973 the production of antibodies soon followed in 1975 by Georges Khler and César Milstein creating monoclonal antibodies.The first commercial monoclonal antibody
The first licensed monoclonal antibody was Orthoclone OKT3, which was approved in 1986. This mouse monoclonal IgG2a antibody detected CD3 antigens. OKT3 was used for preventing kidney transplant rejection by targeting T-lymphocytes presenting CD3.However, production was limited and difficult. Early monoclonal antibody production was initially limited by the availability of suitable myeloma cell lines as well as hybridomas having the risks of being low yielding or genetically unstable. In 2009 it was found that E. coli is an excellent system for expressing antibody fragments and was then utilised to culture single-chain variable regions and antigen-binding fragments of antibodies. However, it has been noticed with using E. coli that synthesis of larger full-sized antibodies may be beyond the capabilities of the small microorganism.
How are monoclonal antibodies produced
The original method of producting monoclonal antibodies was created by injecting an immunogen into an animal, typically goats, mice and rabbits, in which the animal host would have an immune response, and would be regularly monitored. After, the cell fusion process would be carried out, whereby animal B cells are removed and fused with a cancer myeloma cell in order to create an immortalised cell line. This process creates a mono-culture of B-cells all producing the same specific antibody. Each hybridoma produces a singular type of antibody, a monoclonal, which is designated and binds to a specific epitope region of the immunising antigen. Following this, production is scaled up and the monoclonal antibodies are excreted by the B-cells into the cell culture media, where they can be purified for further antigen binging affinity testing and purification.
LEGACY FROM THE 70's. This technique which was established in the 70’s is still used to this day, but with modern advances the process is much faster.
Antibodies of the 21st century
With the vast developments of biochemical and biotechnological techniques, a new method of generating monoclonal antibodies that is utilised greatly in the 21st century is by using phage display.
Phage display
Phage display technique is the isolation of B-lymphocytes from human blood samples and further isolating the mRNA, converting it into cDNA in order to amplify the VH and VL segments.
The principle
These isolated and identified segments are then cloned into a vector next to the PIII protein which will be transfected into E. coli in order to create a library of 1010 cells.
Reproducibility of the process
Once the library is made, the same library can then be used to generate new antibodies and doesn’t have to be remade. No immunisations are required, and the process is done in vitro.
Advantages
This is a much faster way of creating antibodies in contrast to the old traditional method of immunisation of animals, waiting for their B cells to naturally produce the antibodies. A process which can take several months depending on the antibodies.
The future of antibodies
Now, future prospects involve utilising antibodies for various biomedical fields, medicine and therapeutics. Research which includes the use of antibodies has been increasing steadily since the 50’s.

Antibodies in medicine
Within medicine and therapeutic antibodies are now being applied to techniques like:
- Biochemical assays for diagnostics via protein detection
- ELISA diagnostic techniques which use multiple antibodies to detect specific antigens for infectious disease
- analysis of patient antibody profiles
- Monoclonal antibodies are used to treat several diseases such as multiple sclerosis, rheumatoid; arthritis and psoriasis.
Detecting immunuglobins
Antibodies are also developed to detect immunoglobins as some of them can be useful indicators in diagnosis of many ailments and even utilising the detection of human chorionic gonadotropin for pregnancy test kits.
Understanding the immune system
Their resourcefulness has proven to be incredibly useful and the greater understanding of the immune system has come a long way since early established methods of variolation from over a thousand years ago.
Cancer research
Many researchers are looking into the influences antibodies can have on current issues like fighting cancer. There are many studies currently running which are investigating the efficiency of antibody binding for targeting cancerous cells, assisting the bodies immunity in fighting against these harmful cells. In a number of some cases therapies have already undergone clinical trial approval or in market development.
Infectious diseases.
As technology advances and antibodies can be manipulated further and further it is hoped that some of the deadliest pathogenic infections like HIV and Malaria can be eradicated much like the great achievement of globally eradicating smallpox’s from the human population. With their growing involvement within modern medicine, research, therapeutics and diagnostics, antibodies are a versatile resource which we can generate limitlessly with techniques like phage display and cloning.


