| Host: |
E. coli |
| Reactivity: |
Human |
| Note: |
STRICTLY FOR FURTHER SCIENTIFIC RESEARCH USE ONLY (RUO). MUST NOT TO BE USED IN DIAGNOSTIC OR THERAPEUTIC APPLICATIONS. |
| Short Description: |
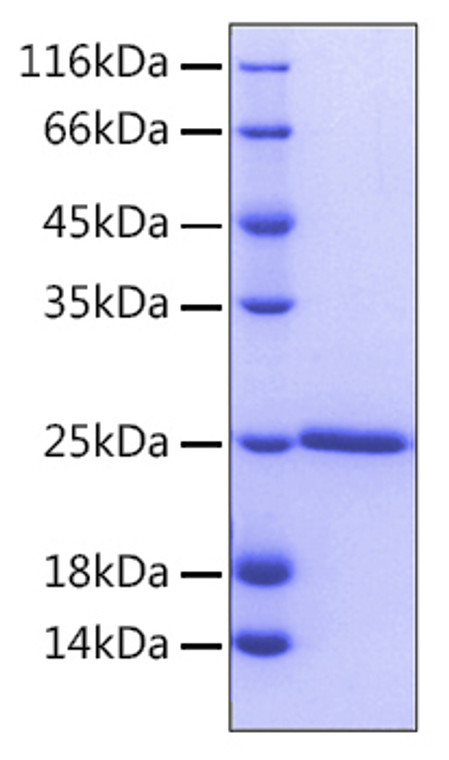
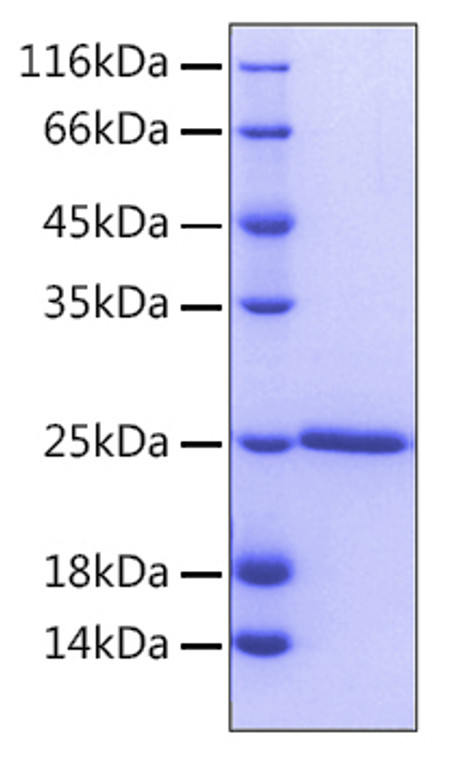
Recombinant-Human Bcl-2-No tag protein was developed from e. coli and has a target region of No tag. For use in research applications. |
| Formulation: |
Supplied in 20mM TRIS, 200mM NaCl, 30% Glycerol, 1mM DTT, pH 8.0. Contact us for customized product form or formulation. |
| Endotoxin: |
Please contact us for more information. |
| Immunoreactivity: |
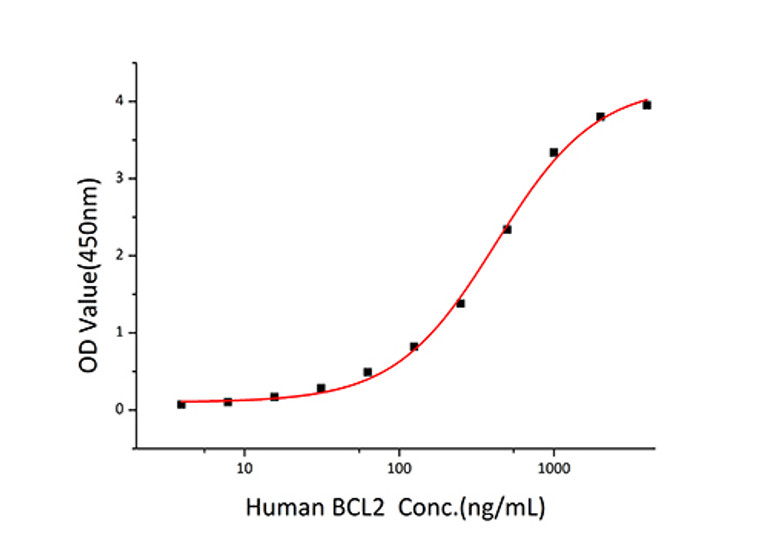
Measured by its binding ability in a functional ELISA. Immobilized Human BCL-XL Protein at 2 Mu g/mL (100 Mu L/well) can bind BCL2 with a linear range of 3.9-421 ng/mL. |
| Gene Symbol: |
BCL2 |
| Gene ID: |
596 |
| Uniprot ID: |
BCL2_HUMAN |
| Immunogen Region: |
Ala2-Asp211 |
| Immunogen: |
Recombinant Human Bcl-2 Protein is produced by E. coli expression system. The target protein is expressed with sequence (Ala2-Asp211) of human BCL2 (Accession #NP_000624.2). |
| Tissue Specificity | Expressed in a variety of tissues. |
| Post Translational Modifications | Phosphorylation/dephosphorylation on Ser-70 regulates anti-apoptotic activity. Growth factor-stimulated phosphorylation on Ser-70 by PKC is required for the anti-apoptosis activity and occurs during the G2/M phase of the cell cycle. In the absence of growth factors, BCL2 appears to be phosphorylated by other protein kinases such as ERKs and stress-activated kinases. Phosphorylated by MAPK8/JNK1 at Thr-69, Ser-70 and Ser-87, wich stimulates starvation-induced autophag. Dephosphorylated by protein phosphatase 2A (PP2A). Proteolytically cleaved by caspases during apoptosis. The cleaved protein, lacking the BH4 motif, has pro-apoptotic activity, causes the release of cytochrome c into the cytosol promoting further caspase activity. Monoubiquitinated by PRKN, leading to an increase in its stability. Ubiquitinated by SCF(FBXO10), leading to its degradation by the proteasome. Ubiquitinated by XIAP, leading to its degradation by the proteasome. |
| Function | Suppresses apoptosis in a variety of cell systems including factor-dependent lymphohematopoietic and neural cells. Regulates cell death by controlling the mitochondrial membrane permeability. Appears to function in a feedback loop system with caspases. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor (APAF-1). Also acts as an inhibitor of autophagy: interacts with BECN1 and AMBRA1 during non-starvation conditions and inhibits their autophagy function. May attenuate inflammation by impairing NLRP1-inflammasome activation, hence CASP1 activation and IL1B release. |
| Protein Name | Apoptosis Regulator Bcl-2 |
| Database Links | Reactome: R-HSA-111447Reactome: R-HSA-111453Reactome: R-HSA-6785807Reactome: R-HSA-844455Reactome: R-HSA-9018519Reactome: R-HSA-9634638 |
| Cellular Localisation | Mitochondrion Outer MembraneSingle-Pass Membrane ProteinNucleus MembraneEndoplasmic Reticulum MembraneCytoplasm |
| Alternative Protein Names | Apoptosis Regulator Bcl-2 proteinBCL2 protein |
Information sourced from Uniprot.org
12 months for antibodies. 6 months for ELISA Kits. Please see website T&Cs for further guidance