| Host: |
Rabbit |
| Applications: |
WB/ICC |
| Reactivity: |
Mouse/Rat/Zebrafish/Bovine/Human/Xenopus |
| Note: |
STRICTLY FOR FURTHER SCIENTIFIC RESEARCH USE ONLY (RUO). MUST NOT TO BE USED IN DIAGNOSTIC OR THERAPEUTIC APPLICATIONS. |
| Short Description: |
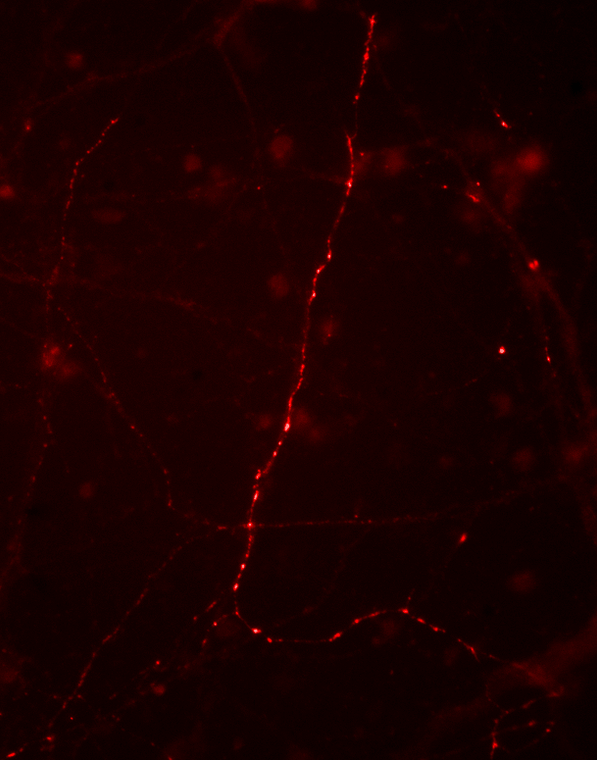
Rabbit polyclonal antibody anti-Phospho-Synapsin I-Ser9 is suitable for use in Western Blot and Immunocytochemistry research applications. |
| Clonality: |
Polyclonal |
| Conjugation: |
Unconjugated |
| Isotype: |
IgG |
| Formulation: |
100 µl in 10 mM HEPES (pH 7.5) , 150 mM NaCl, 100 µg per ml BSA and 50% Glycerol. |
| Purification: |
This antibody was antigen affinity purified from pooled serum. |
| Dilution Range: |
WB 1:1000IHCICC 1:500IP |
| Storage Instruction: |
Store at-20°C for up to 1 year from the date of receipt, and avoid repeat freeze-thaw cycles. |
| Immunogen: |
Synthetic phospho-peptide corresponding to amino acid residues surrounding Ser9 conjugated to KLH. |
| Background | Synapsin I plays a key role in synaptic plasticity in brain (Feng et al., 2002; Nayak et al., 1996). This effect is due in large part to the ability of the synapsins to regulate the availability of synaptic vesicles for release. In addition to its role in plasticity, the expression of synapsin I is a precise indicator of synapse formation (Moore and Bernstein, 1989; Stone et al., 1994). Thus, synapsin I immunocytochemistry provides a valuable tool for the study of synaptogenesis. The role of synapsin in synaptic plasticity and in synaptogensis is regulated by phosphorylation (Jovanovic et al., 2001; Kao et al., 2002). Serine 9 is the site on synapsin I that is phosphorylated by cAMP-dependent protein kinase and by calcium calmodulin kinase I (Czernik et al., 1987). Phosphorylation of this site is thought to Western blot of rat cortical lysate showing specific regulate synaptic vesicle function and neurite outgrowth (Kao et al., immunolabeling of the ~78 kDa synapsin I phosphorylated at 9 2002). Ser in the first lane (-). Phosphospecificity is shown in the second lane (+) where the immunolabeling is completely eliminated by blot treatment with lambda phosphatase ( Lambda- |
Information sourced from Uniprot.org
12 months for antibodies. 6 months for ELISA Kits. Please see website T&Cs for further guidance