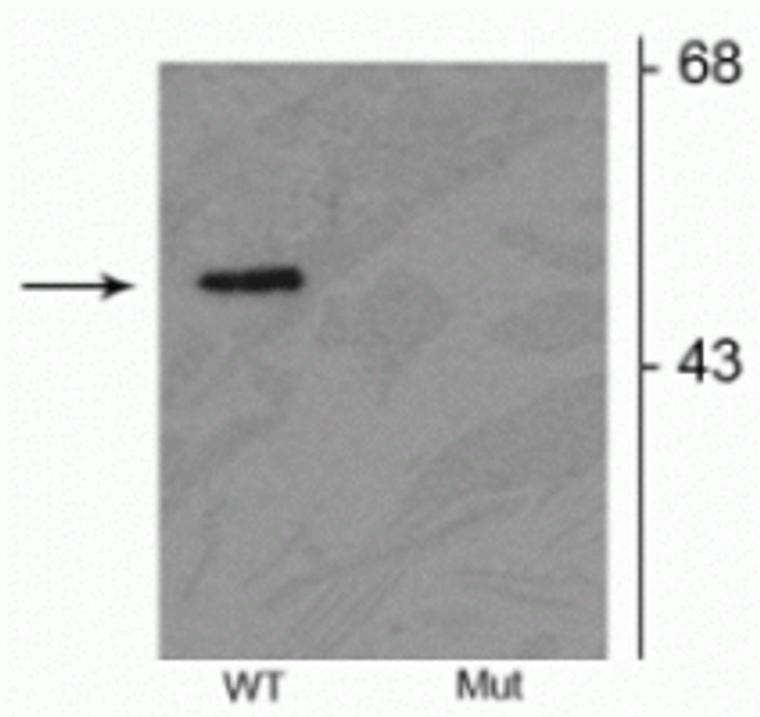
| Function | Functions within a multiprotein E3 ubiquitin ligase complex, catalyzing the covalent attachment of ubiquitin moieties onto substrate proteins. Substrates include SYT11 and VDAC1. Other substrates are BCL2, CCNE1, GPR37, RHOT1/MIRO1, MFN1, MFN2, STUB1, SNCAIP, SEPTIN5, TOMM20, USP30, ZNF746, MIRO1 and AIMP2. Mediates monoubiquitination as well as 'Lys-6', 'Lys-11', 'Lys-48'-linked and 'Lys-63'-linked polyubiquitination of substrates depending on the context. Participates in the removal and/or detoxification of abnormally folded or damaged protein by mediating 'Lys-63'-linked polyubiquitination of misfolded proteins such as PARK7: 'Lys-63'-linked polyubiquitinated misfolded proteins are then recognized by HDAC6, leading to their recruitment to aggresomes, followed by degradation. Mediates 'Lys-63'-linked polyubiquitination of a 22 kDa O-linked glycosylated isoform of SNCAIP, possibly playing a role in Lewy-body formation. Mediates monoubiquitination of BCL2, thereby acting as a positive regulator of autophagy. Protects against mitochondrial dysfunction during cellular stress, by acting downstream of PINK1 to coordinate mitochondrial quality control mechanisms that remove and replace dysfunctional mitochondrial components. Depending on the severity of mitochondrial damage and/or dysfunction, activity ranges from preventing apoptosis and stimulating mitochondrial biogenesis to regulating mitochondrial dynamics and eliminating severely damaged mitochondria via mitophagy. Activation and recruitment onto the outer membrane of damaged/dysfunctional mitochondria (OMM) requires PINK1-mediated phosphorylation of both PRKN and ubiquitin. After mitochondrial damage, functions with PINK1 to mediate the decision between mitophagy or preventing apoptosis by inducing either the poly- or monoubiquitination of VDAC1, respectively.polyubiquitination of VDAC1 promotes mitophagy, while monoubiquitination of VDAC1 decreases mitochondrial calcium influx which ultimately inhibits apoptosis. When cellular stress results in irreversible mitochondrial damage, promotes the autophagic degradation of dysfunctional depolarized mitochondria (mitophagy) by promoting the ubiquitination of mitochondrial proteins such as TOMM20, RHOT1/MIRO1, MFN1 and USP30. Preferentially assembles 'Lys-6'-, 'Lys-11'- and 'Lys-63'-linked polyubiquitin chains, leading to mitophagy. The PINK1-PRKN pathway also promotes fission of damaged mitochondria by PINK1-mediated phosphorylation which promotes the PRKN-dependent degradation of mitochondrial proteins involved in fission such as MFN2. This prevents the refusion of unhealthy mitochondria with the mitochondrial network or initiates mitochondrial fragmentation facilitating their later engulfment by autophagosomes. Regulates motility of damaged mitochondria via the ubiquitination and subsequent degradation of MIRO1 and MIRO2.in motor neurons, this likely inhibits mitochondrial intracellular anterograde transport along the axons which probably increases the chance of the mitochondria undergoing mitophagy in the soma. Involved in mitochondrial biogenesis via the 'Lys-48'-linked polyubiquitination of transcriptional repressor ZNF746/PARIS which leads to its subsequent proteasomal degradation and allows activation of the transcription factor PPARGC1A. Limits the production of reactive oxygen species (ROS). Regulates cyclin-E during neuronal apoptosis. In collaboration with CHPF isoform 2, may enhance cell viability and protect cells from oxidative stress. Independently of its ubiquitin ligase activity, protects from apoptosis by the transcriptional repression of p53/TP53. May protect neurons against alpha synuclein toxicity, proteasomal dysfunction, GPR37 accumulation, and kainate-induced excitotoxicity. May play a role in controlling neurotransmitter trafficking at the presynaptic terminal and in calcium-dependent exocytosis. May represent a tumor suppressor gene. |